BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar

Current state of U.S. Food and Drug Administration regulation for cellular and gene therapy products: potential cures on the horizon - Cytotherapy

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development
Novartis fills manufacturing gap for CAR-T therapy Kymriah with first Asian production facility | Fierce Pharma

Industry's Giant Leap Into Cellular Therapy: Catalyzing Chimeric Antigen Receptor T Cell (CAR-T) Immunotherapy | SpringerLink

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
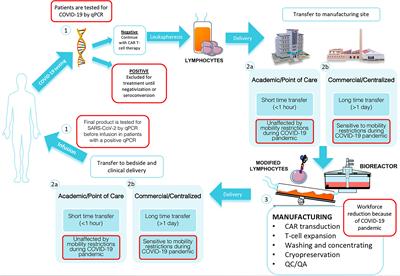
Frontiers | Manufacturing and Management of CAR T-Cell Therapy in “COVID-19's Time”: Central Versus Point of Care Proposals

How to cover Novartis' $475K CAR-T drug Kymriah? A 'new payment model' is the only way, Express Scripts says | Fierce Pharma

PDF) Chimeric Antigen Receptor-T Cell Therapy: Practical Considerations for Implementation in Europe

Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma | Nature Communications
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar







